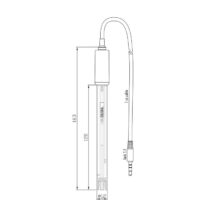
Description
Hanna Instruments offers a wide variety of pH electrodes that are designed for many different applications. The type of glass used for sensing pH, bulb shape, body material, type of junction, type of reference and electrolyte used are just some of the design considerations.
The HI1053P uses low temperature (LT) glass, conical bulb, glass body, triple ceramic junction and is refillable with 3.5M KCl.
Low Temperature Glass Formulation
The measurement of pH at very high temperatures is detrimental to the sensitive glass bulb and will shorten the life of it. A pH electrode with general purpose (GP) glass will have a resistance of 100 megaohms at 25℃ while the resistance of LT glass is around 50 megaohms at 25℃. As the temperature of the glass decreases in the sample, the resistance of the LT glass will approach that of GP glass. If using GP glass, the resistance would increase above the optimum range, resulting in increased impedance and ultimately affecting the measurement. The HI1053B is suitable to use with samples that measure from -5 to 100℃.
Conical Glass Tip
The conical shaped tip design allows for penetration into solids, semi solids, and emulsions for the direct measurement of pH in food products including meat, cheese, yogurt, and milk.
Glass Body
The glass body is ideal for laboratory use. The glass is resistant to many harsh chemicals and is easily cleaned. The glass body also allows for a fast transfer of heat to the internal reference electrolyte. The mV generated by the reference cell is temperature dependent. The faster the equilibrium the steadier the reference potential.
Triple Ceramic Junction
The triple ceramic junction allows a faster flow rate of electrolyte from the reference into the solution. A standard pH electrode will use a single ceramic junction that allows for 15 to 20 µL/hour of electrolyte to flow; the HI1053P has three ceramic junctions, providing for 40 to 50 µL/hour of electrolyte to flow. This high flow rate provides faster electrode response and more stable measurement in viscous solutions or samples of low conductivity, such as pure water, where a long stabilization time is often observed.
Double Junction Reference
A double junction electrode has an internal compartment surrounding the reference wire. Silver ions are present in the electrolyte of the internal compartment, which houses the Ag/AgCl reference wire; the electrolyte outside this compartment is silver free. The double junction design means that virtually no silver from the electrode enters the sample. This design allows measurement in applications where silver ions in the sample are undesirable or silver precipitates on the junction are likely to form.
Refillable
The HI1053P is a refillable probe. Since it is a double junction pH electrode, the fill solution is the HI7082 3.5M KCl. This solution does not contain any silver as with single junction electrode fill solutions. The absence of silver will prevent any silver precipitate from forming at the junction surface and clogging it. Clogging of the junction will result in drifty and erratic readings.
BNC + Pin Connector
The HI1053P has a BNC and pin connector. The BNC connector is universal in that it can be used on any pH meter that has the female BNC probe input. The pin connector is used to enable the CAL Check function on the following benchtop pH meters; HI122, HI123, HI221, HI222, HI223, HI2221, HI2222, and HI2223.

Conventional electrodes are normally single junction. As depicted by the figure above, these electrodes have only a single junction between the internal refernce wire and the external solution. Under adverse conditions, such as high pressure, high temperature, highly acidic or alkaline solutions, the positive flow of the electrolyte through the junction is often reversed resulting in the ingress of sample solution into the reference compartment. If this is left unchecked, the reference electrode can become contaminated, leading to complete electrode failure. Another potential problem with single junction electrodes is the clogging of the junction due to silver chloride (AgCl) precipitation. Silver can be easily precipitate in samples that contain Tris buffer or heavy metals. When the electrolyte solution makes contact with the sample, some AgCl will precipitate on the external face of the junction. The result is drifty readings obtained from the sensor.
Hanna’s double junction system, as the name implies, has two junctions, only one of which is in contact with the sample as shown in the figure. Under adverse conditions, the same tendency of sample ingress is evident. However, as the reference electrode system is separated physically from the intermediate electrolyte area, the contamination of the electrode is minimized. The likelihood of clogging of the junction is also reduced with a double junction electrode since the outer reference cell uses a fill solution that is “silver-free”. Since there is no silver present, there is no precipitate forming to clog the junction.